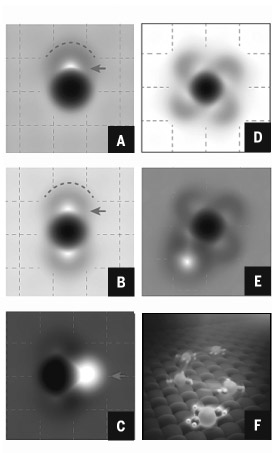
In a simulation using atomic force microscopy, four water molecules bond with a sodium ion one by one (A to D). A fifth water molecule (white spot at lower left) bonds with the hydrated sodium ion. As one sodium ion can only bond with four water molecules tightly at most, the fifth one can only bond outside. (E) An artist's rendering of a hydrated sodium ion with three water molecules. (F)
Chinese scientists have become the first to directly observe the atomic structure of a hydrated sodium ion-the basic chemical makeup of seawater.
The technology can be used to study other water-based liquids, opening new avenues for molecular and materials sciences, experts said on Monday in the science journal Nature.
It is the first time scientists have been able to visualize the atomic structure of hydrated ions in their natural environment since the notion was proposed more than a hundred years ago.
The same team of scientists also discovered that exactly three water molecules are needed to allow a single sodium ion to travel 10 to 100 times faster than other ion hydrates-a process that could lead to more efficient ion batteries, anti-corrosion coatings and seawater desalination plants, according to the Nature article.
Water is the most plentiful liquid on Earth. Its simple chemical structure-two hydrogen atoms bonded to one oxygen atom-is the basic building block of most life on Earth, said Wang Enge, a physicist and academician of the Chinese Academy of Sciences.
"But the science behind water, especially regarding its structure and interaction with other chemicals, is extremely hard and not well understood," Wang said. In 2005, the journal Science listed the structure of water as one of the most compelling scientific puzzles, despite a century's worth of research having been done.
Since the late 19th century, scientists have been studying ion hydration, a process in which water dissolves soluble materials such as sodium chloride, or salt. Although the process is extremely common in nature, exactly how it works at an atomic level has remained a mystery.
"The main reason for water's complexity is its simplicity," said Jiang Ying, a professor at Peking University's International Center for Quantum Materials, who was part of the study.
Because hydrogen atoms are so simple and small compared with the oxygen atom, the weird properties of quantum mechanics start to interfere with experiments and make them less predictable, he said.
"Therefore, it is crucial for scientists to directly see how water interacts with other materials at an atomic level." By using new atomic force microscopy developed by Chinese scientists, it's possible "to see even the smallest changes in a single water molecule's structure around the ions", Jiang said.
Scientists found that three water molecules surrounding a single sodium ion can travel exceptionally fast on a sodium chloride molecule's surface. This "sublime phenomenon" can occur at room temperature, but also applies with other chemical ions such as potassium ions-one of the key ions necessary for neural cell communication.
"Although the magic number for each type of ion might be different, the phenomenon is a game changer for ion-related fields," he said. For example, engineers can alter the flow speed of lithium ions in batteries to make them charge faster or store more power.
Scientists can also create special filter systems that can change the number of water molecules surrounding an ion, thus speeding up or reducing the filtering speed according to specific needs.
This discovery also allows scientists to have a better understanding of how cells communicate with each other by exchanging ions through channels on their membranes, Jiang said.
This has potentially profound scientific implications for future applications in biology and medicine, he said, adding that two Nobel Prizes were given to research related to ion channels in the last two decades-one for their discovery in 1991 and the other for their mechanisms in channeling water in 2003.