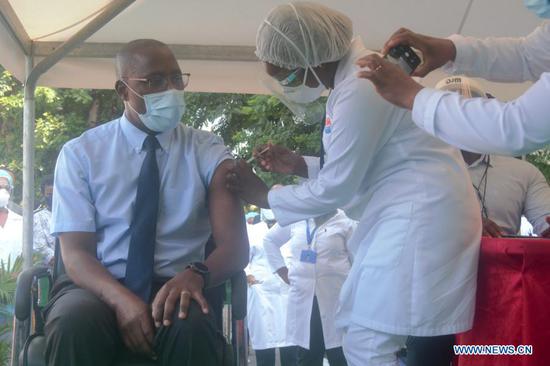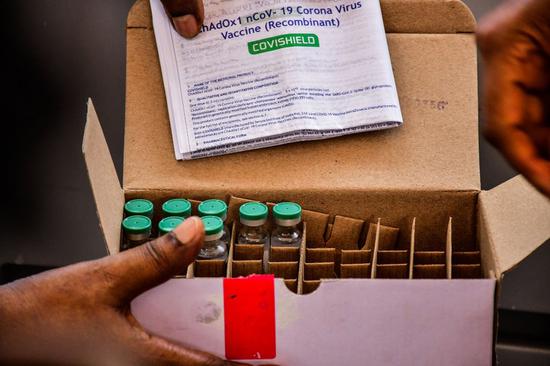
Doses of AstraZeneca COVID-19 vaccine are displayed during the launch of the COVID-19 vaccination campaign at Mulago Specialized Women and Neonatal Hospital in Kampala, Uganda, March 10, 2021. (Photo by Joseph Kiggundu/Xinhua)
Head of the World Health Organization (WHO) said on Friday that the Geneva-based UN health agency "systematically reviews safety signals, and is carefully assessing the current reports on the AstraZeneca vaccine."
The COVID-19 vaccine developed by AstraZeneca and the University of Oxford has been suspended in a number of countries across Europe and Asia, following reports of blood clots in some vaccinated people.
"WHO is aware that some countries have suspended the use of AstraZeneca vaccines, based on reports of blood clots in some people who received doses of the vaccine from two batches. This measure was taken as a precaution while a full investigation is finalized," said WHO Director-General Dr. Tedros Adhanom Ghebreyesus at a press briefing on Friday.
A number of countries like Denmark, Norway, Iceland, Romania and Thailand have suspended the rollout of the AstraZeneca/Oxford vaccine after it was linked to blood clots in recipients, while Austria and France have decided to continue using it.
"It is important to note that the European Medicines Agency has said there is no indication of a link between the vaccine and blood clots, and that the vaccine can continue to be used while its investigation is ongoing," Tedros said.
The European Commission said on Thursday that the AstraZeneca vaccine is still safe to use, adding that the European Medicines Agency (EMA) had adopted a preliminary review on the case in Austria "where they said there is no specific indication that the vaccination led to these conditions."
"As soon as WHO has gained a full understanding of these events, the findings and any changes to our current recommendations will be communicated immediately to the public," Tedros said.