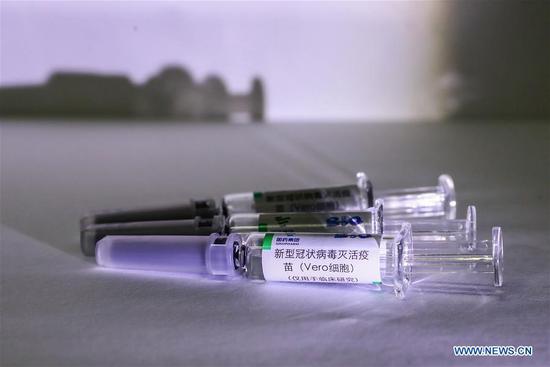
China approved clinical trials for two types of inactivated vaccines for COVID-19 on Tuesday, announced China's Joint Prevention and Control Mechanism of the State Council.
The vaccines are developed by Wuhan Institute of Biological Products of China National Pharmaceutical Group and a Beijing-based unit of Sinovac Biotech.
According to the World Health Organization, inactivated vaccines are made from microorganisms (viruses, bacteria, other) that have been killed through physical or chemical processes. After immunization, the vaccine antigens cannot replicate in the vaccinated person or cause disease.
Chinese scientists have been racing to develop COVID-19 vaccines via five approaches – inactivated vaccines, genetic engineering subunit vaccines, adenovirus vector vaccines, nucleic acid vaccines, and vaccines using attenuated influenza virus as vectors.
The adenovirus vector vaccine against COVID-19 developed by China's Academy of Military Medical Sciences has previously been approved and is currently in the second phase of clinical trials.