Staff members talk beside the virus culture/inactivation area of a vaccine production plant of China National Pharmaceutical Group (Sinopharm) in Beijing, capital of China, April 10, 2020. China has approved two COVID-19 inactivated vaccine candidates for clinical trials, according to the State Council joint prevention and control mechanism against the coronavirus Tuesday. The two vaccine candidates are developed by Wuhan Institute of Biological Products under the China National Pharmaceutical Group (Sinopharm) and Sinovac Research and Development Co., Ltd, a company based in Beijing. Clinical trials of the two vaccines have started. (Xinhua/Zhang Yuwei)
China has approved two COVID-19 inactivated vaccine candidates for clinical trials, according to the State Council joint prevention and control mechanism against the coronavirus Tuesday.
The two vaccine candidates are developed by Wuhan Institute of Biological Products under the China National Pharmaceutical Group (Sinopharm) and Sinovac Research and Development Co., Ltd, a company based in Beijing. Clinical trials of the two vaccines have started.

A staff member tests samples of the COVID-19 inactivated vaccine at a vaccine production plant of China National Pharmaceutical Group (Sinopharm) in Beijing, capital of China, April 11, 2020. China has approved two COVID-19 inactivated vaccine candidates for clinical trials, according to the State Council joint prevention and control mechanism against the coronavirus Tuesday. The two vaccine candidates are developed by Wuhan Institute of Biological Products under the China National Pharmaceutical Group (Sinopharm) and Sinovac Research and Development Co., Ltd, a company based in Beijing. Clinical trials of the two vaccines have started. (Xinhua/Zhang Yuwei)
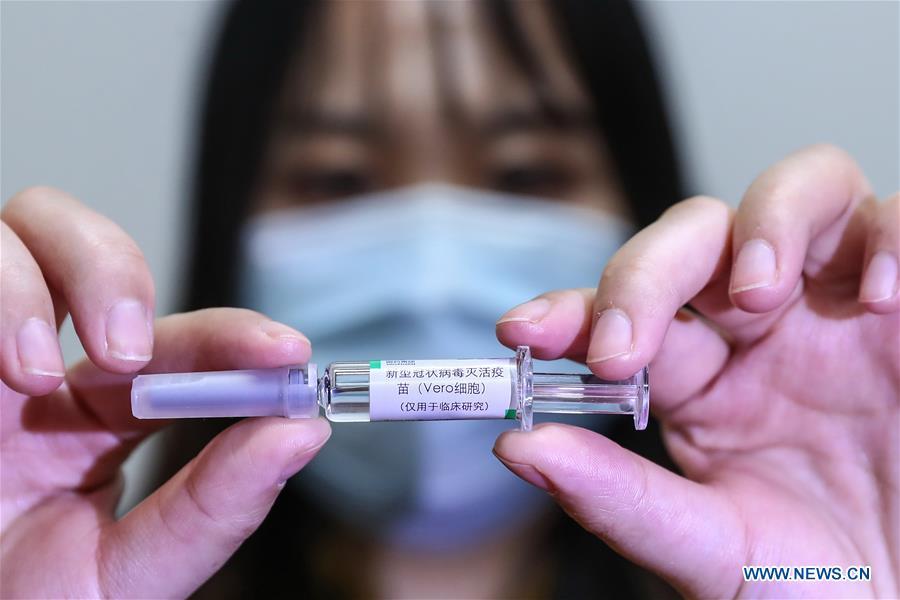
A staff member displays a sample of the COVID-19 inactivated vaccine at a vaccine production plant of China National Pharmaceutical Group (Sinopharm) in Beijing, capital of China, April 10, 2020. China has approved two COVID-19 inactivated vaccine candidates for clinical trials, according to the State Council joint prevention and control mechanism against the coronavirus Tuesday. The two vaccine candidates are developed by Wuhan Institute of Biological Products under the China National Pharmaceutical Group (Sinopharm) and Sinovac Research and Development Co., Ltd, a company based in Beijing. Clinical trials of the two vaccines have started. (Xinhua/Zhang Yuwei)
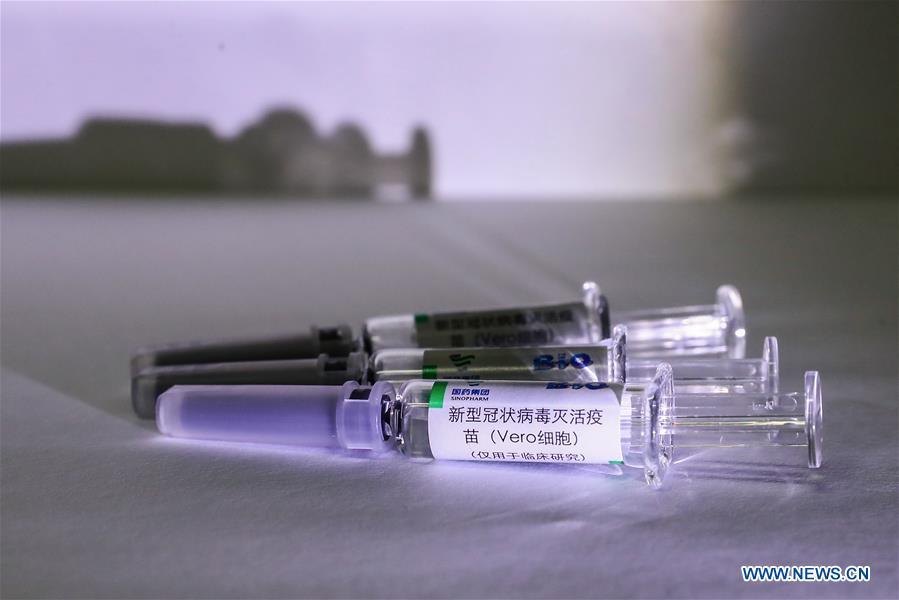
Samples of the COVID-19 inactivated vaccine are seen at a vaccine production plant of China National Pharmaceutical Group (Sinopharm) in Beijing, capital of China, April 10, 2020. China has approved two COVID-19 inactivated vaccine candidates for clinical trials, according to the State Council joint prevention and control mechanism against the coronavirus Tuesday. The two vaccine candidates are developed by Wuhan Institute of Biological Products under the China National Pharmaceutical Group (Sinopharm) and Sinovac Research and Development Co., Ltd, a company based in Beijing. Clinical trials of the two vaccines have started. (Xinhua/Zhang Yuwei)