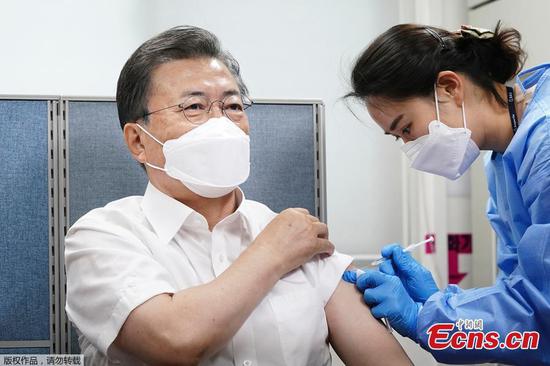
China's health authorities issued the first official COVID-19 vaccination guidelines on Monday, suggesting seniors aged 60 and above get inoculated as current clinical research data showed that vaccination is safe for senior citizens. It also noted COVID-19 vaccine booster shots are not recommended for now.
The guidelines were released by the National Health Commission after the number of vaccination shots in the country reached 100 million as of Sunday.
China has authorized five COVID-19 vaccines for conditional marketing or emergency use, including three inactivated vaccines, one adenovirus vector vaccine, and one recombinant protein sub-unit vaccine. The safety of all these vaccines has been proven, it noted.
The guidelines suggested seniors aged 60 and above should get vaccinated, saying that the data from Phase I and Phase II clinical studies of four conditionally approved vaccines showed that vaccination is safe for this group, even though there is no data on the protective efficacy of the vaccines yet.
Compared with people aged 18-59, the neutralizing antibody titer of senior citizens after vaccination is slightly lower, but the seroconversion rate of neutralizing antibodies is similar, said the guidelines.
As China rolled out its vaccination campaign with increased speed across the country, many cities, such as Beijing and Shanghai, have started vaccinating some elderly people and patients with chronic diseases who are willing to be vaccinated and are in a good physical condition, on the premise of fully assessing their health status and infection risk.
Feng Duojia, president of the China Vaccine Industry Association, told the Global Times previously that the inactivated COVID-19 vaccines from Sinopharm and Sinovac have been approved for use in aged people in Beijing. The Global Times also learned that the adenovirus vector vaccine produced by CanSino has included people 60 and over for its applicable vaccination age when it was approved and marketed conditionally.
The guidelines also noted that booster shots are not recommended at this stage.
An immunologist reached by the Global Times previously said that China should focus on getting more people vaccinated and protected at the current stage as the supply of COVID-19 vaccines are still in short.
Although booster shots are still not suggested, Zhang Yuntao, a vice president and chief scientist of China National Biotec Group, a Sinopharm subsidiary, said at a press conference on Sunday that the group has designed COVID-19 booster shots to effectively improve the durability of antibodies. The company said initial results showed booster shots can improve vaccines' efficacy against virus variants.
The guidelines also noted it is not recommended for people under the age of 18 to get vaccinated for now.
As for whether the COVID-19 vaccines from different manufacturers can be used in combination, the guidelines recommended completing the vaccination with the same vaccine product. Under special circumstances, people can use vaccine products from other manufacturers of the same type to complete the inoculation.