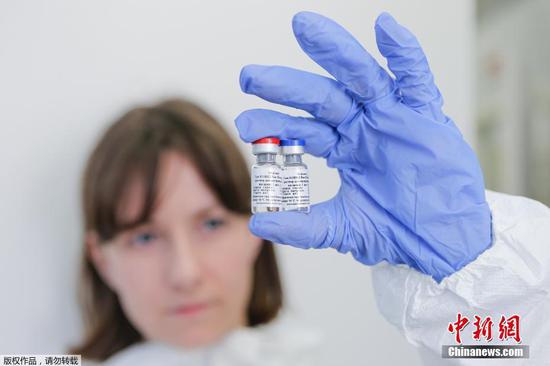
An inactivated COVID-19 vaccine developed by China has started phase-3 clinical trials in Argentina, according to its developer China National Biotec Group (CNBG), which is affiliated to Sinopharm.
The launch ceremony of the phase-3 clinical trials was held in Beijing Friday after the CNBG obtained the certificate of approval for the process.
This is an achievement brought about by CNBG's international cooperation in a bid to develop a COVID-19 vaccine. The inactivated vaccine received approval for phase-3 clinical trials in the United Arab Emirates on June 23, and in Peru and Morocco on Thursday.
The CNBG will work together with a company in Argentina to promote research and development related to the inactivated vaccine.
Liu Jingzhen, chairman of Sinopharm, said the phase-3 clinical trials will help fight the epidemic and contribute to the building of a community of health for all.