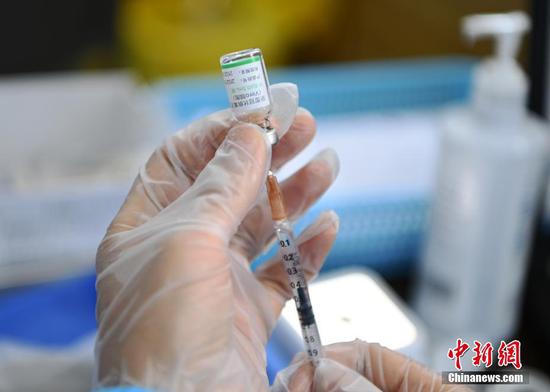
A medical worker prepares a dose of COVID-19 vaccine at a vaccination site in Chengdu, southwest China's Sichuan Province, May 11, 2021. (Photo/China News Service)
(ECNS) -- A COVID-19 vaccine manufactured by China’s CanSino Biologics was added to the emergency use listing released by the World Health Organization (WHO), the organization announced on Thursday.
This is the 3rd Chinese vaccine authorized by the WHO for emergency use following Sinopharm and Sinovac Biotech, as well as the 11th such product in the world validated by the organization.
The WHO recommends use of the vaccine as a single (0.5ml) dose, in all age groups 18 and above.
The vaccine was found to have a 64 percent efficacy against symptomatic disease and 92 percent efficacy in preventing severe cases.
















































 京公网安备 11010202009201号
京公网安备 11010202009201号