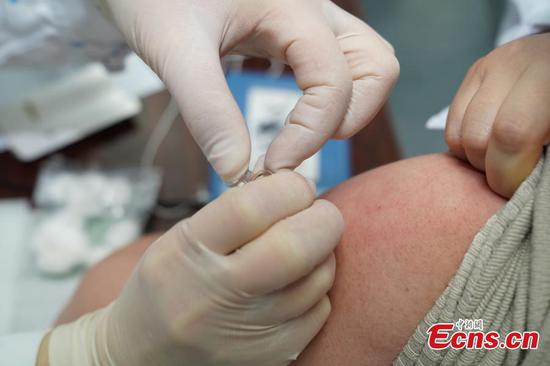
Beijing-based Sinovac Life Sciences Co., Ltd. on Wednesday filed an application with Chinese authorities for conditional marketing authorization of its anti-COVID-19 vaccine CoronaVac.
The application has been received by the National Medical Products Administration (NMPA), according to Sinovac Biotech Ltd., which has a majority stake in the vaccine producer.
The inactivated vaccine CoronaVac has made progress in phase three clinical trials overseas, showing a good level of safety after inoculation, according to a company statement.
Fourteen days after the inoculation of two doses of CoronaVac, the effectiveness of the vaccine in protecting against diseases caused by COVID-19 has met the relevant technical standards laid out by the World Health Organization and the requirements of the NMPA, the statement said.