
British pharmaceutical giant GlaxoSmithKline (GSK) has been investigating its employees in China and those found to have broken company compliance rules will be warned or even dismissed, the company said in a statement -e-mailed to the Global Times Thursday.
The statement comes nine months after GSK was caught in a bribery scandal in China, in which media reports said around 3 billion yuan ($483 million) had been funneled to travel agencies to facilitate bribes such as overseas travel packages to doctors.
"Employees that are found to have violated company policies may be warned or dismissed depending on the extent of the violation," the company said in the e-mail.
GSK did not disclose how many employees it has dismissed, but 21st Century Business Herald newspaper reported on Thursday that around 1,000 medical representatives have been found to have made possible compliance violations and over 150 employees have been laid off so far, including sales management staff.
Citing a GSK medical representative who declined to be named, the report said that GSK withheld some of the bonuses of the dismissed medical representatives, and also said they could not get reimbursement for their previous sales expenses.
On March 31, the dismissed medical representatives launched a demonstration in front of GSK's China headquarters in Shanghai, protesting against GSK's withholding of their bonuses and the layoffs.
In the e-mail statement, GSK admitted that some of the incentive payments for employees who are subject to a compliance-related investigation may be withheld.
"GSK is currently investigating potential irregularities related to certain 2013 expense submissions to ensure that they were made in accordance with both GSK policies and values," the company said.
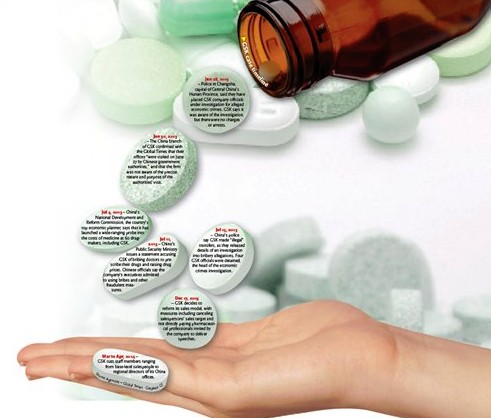
GSK had a tough year in 2013. On June 27, some of the company's China offices were visited by government authorities and later China's Ministry of Public Security confirmed that the company had been investigated for "serious economic crimes."
Other multinational pharmaceutical firms have also had bribery scandals exposed after GSK's bribery issue. Companies including Sanofi-Aventis, Novo Nordisk and AstraZeneca were said to have bribed doctors to boost sales.
Zhong Hongyue, a pharmaceutical analyst at consulting firm Frost & Sullivan, said that medical representatives have been very cautious in contacting doctors recently, and even the number of legitimate promotion events has been reduced.
In December, GSK announced that it would cancel sales target for individual medical representatives and it would gradually stop providing payment to medical professionals invited to its medical conferences.
Affected by the bribery scandal, GSK's China pharmaceuticals and vaccines sales plunged 61 percent year-on-year in the third quarter of 2013, and the decline eased to an 18 percent year-on-year drop in the fourth quarter, according to GSK's financial results released in February.
The lack of occupational standards for medical representatives has been the main reason behind these irregularities, according to Zuo Yuzeng, an official at R&D-based Pharmaceutical Association Committee (RDPAC), which represents over 30 multinational pharmaceutical companies in China.
"Unqualified medical representatives are mixed up with qualified professionals, but it was those unqualified ones who have left a deep impression on the public," Zuo told the Global Times Thursday.
Zuo noted that medical representatives serve as an important channel to deliver and collect medical information between doctors and drug makers, thus the "threshold for the occupation should be raised."
Since 2006 medical representatives from most of the association's member companies have had to pass an examination organized by the association, which tests the representatives' ethical and medical knowledge, Zuo said.
Top city health planner‘s arrest linked to GSK bribery scandal
2013-12-19GSK revamps sales reps‘ compensation
2013-12-19China might charge some local GSK execs: report
2013-11-05GSK China bribe suspect is captured
2013-10-09GSK in ‘constructive’ talks over graft scandal
2013-07-23GSK case does not mean change in foreign investment policy
2013-07-19Copyright ©1999-2018
Chinanews.com. All rights reserved.
Reproduction in whole or in part without permission is prohibited.